standardize sodium thiosulfate
Standardisation of 01N sodium thiosulphate Take 10 ml of Potassium Iodate solution Add 2 gm of Potassium Iodide and 5 ml of dilute H 2 SO 4keep it in dark for 10 minutes add 2 to 3 drops of starch indicator andtitrate with sodium thiosulphte using starch solution as indicator until the blue colour is disappeared. Hydrochloric acid is added.

0 005 M Sodium Thiosulfate Standard Solution Dilute 50 Cm3
Immediately add 5 mL starch indicator and continue to.
. Potassium iodate a strong oxidizing agent is treated with excess potassium iodide in acidic media which liberates iodine which is back titrated with. First sodium bicarbonate is added to a iodate-free solution of potassium iodide. This method is developed using an automated titration system Excellence Titrator.
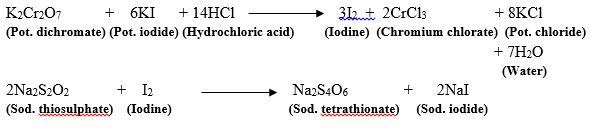
01 N sodium thiosulphate Potassium iodate Potassium iodide PRINCIPLE. A 01 M sodium thiosulphate. Standardization of sodium thiosulfate using potassium dichromate.
Here is my titration to standardize my thiosulfate solutionNote that I went past the endpoint by 1 drop. Sodium thiosulfate is used in gold mining water treatment analytical chemistry the. The mixture is shaken until the salts dissolve then conc.
Add 2 drops of CHCl3. To do so he or she will react it with a solution of iodine. Typically it is available as the white or colorless pentahydrate Na 2 S 2 O 3 5H 2 OThe solid is an efflorescent loses water readily crystalline substance that dissolves well in water.
Day 1 - Standardization of Sodium Thiosulfate Solution TAs Preparation of 0025XX M sodium thiosulfate solution 12 The TAs will prepare a solution of approximately 0025XX M Na 2 S 2 O 3 as follows. Clean the washed burette with the approximately 01 mol dm-3 standardised sodium thiosulphate which is going to be prepared. Dissolve 0200 g of potassium bromate weighed accurately in sufficient water to produce 2500 ml.
Prepare this reagent in a fume cupboard. This is a common situation in the lab practice. Sodium thiosulfate is a chemical and medication.
In the standardization iodine triiodide liberated by potassium dichromate in an acidic potassium iodide solution is titrated with a sodium thiosulfate solution. What is the use of adding sodium. 29 Chloroform CHCl3 AR.
Introduction Sample Preparation and Procedures Chemistry Instruments and Accessories Method in Detail. 210 Standard sodium thiosulphate solution 0025 N. 13 Add 15 mL of 6N NaOH to slow down bacterial decomposition and dilute the.
Add 15 mL 6 N NaOH or 04 g solid NaOH and dilute to 1 L. Sodium thiosulfate sodium thiosulphate is an inorganic compound with the formula Na 2 S 2 O 3. Introduction Sample Preparation and Procedures Chemistry Instruments and Accessories Method in Detail.
The only problem is selection of the volume of thiosulfate sample. To standardise 0025 N sodium thiosulfate. Hence the reaction is stoichiometric and can be used to estimate the concentration of the oxidizing agent.
The amount of thiosulfate required for the reducing the liberated iodine is directly proportional to the amount of oxidizing agent. The iodine liberation process significantly affects the titration results. Once it has completely dissolved make up the volume to 1000 ml.
Take 248 g of sodium thiosulphate Na2O3S2 and dissolve in 200 ml of distilled water in a volumetric flask and properly mixing it. Dissolve 25 g of sodium thiosulphate and 02 g of sodium carbonate in carbon dioxide-free water and dilute to 1000 ml with the same solvent. SODIUM THIOSULFATE CAN BE STANDARDIZED AGAINST PURE IODINE DISSOLVED IN KI OR AGAINST IODINE SET FREE FROM AN ACIDIFIED SOLUTION OF KI BY STANDARD SOLUTION OF POTASSIUM PERMANGANATE OR VARIOUS PRIMARY STANDARD OXIDIZING AGENTS SUCH AS POTASSIUM IODATE.
Typically it is available as the white or colorless pentahydrate Na₂S₂O₃5H₂O. This method is developed using an automated titration system Excellence Titrator. Preparation of 01 N potassium iodate.
It is an inorganic compound with the formula Na₂S₂O₃xH₂O. As a medication it is used to treat cyanide poisoning and pityriasis versicolor. Take 100 mL distilled water in conical flask Add 2g of Potassium di-iodide Add 10 mL of potassium di chromate And lastly add 2 mL of Sulfuric acid Now.
X H 2 O. The solid is an efflorescent crystalline substance that dissolves well in water. If we have iodine solution of known concentration we can easily use it as a standard for thiosulfate solution standardization and vice versa.
5H2O and dissolve it in 800 mL of hot distilled water. The standard solution of the sodium thiosulfate is used to reduce the iodine to iodide. In this process first we need to liberate iodine to react with sodium thiosulfate.
ASTM D1510-16 also describes the standardization method for sodium thiosulfate solution using KIO 3 KI as a primary standard. Sodium thiosulfate is often standardized with potassium dichromate. Dissolve 6205 g Na2S2O35H2O in reagent grade water.
Leaving Cert Chemistry- By kind permission of Folens. The principle of standardization of sodium thiosulphate is based on redox iodometric titration with potassium iodate as primary standard. ASTM D1510-16 also describes the standardization method for sodium thiosulfate solution using KIO 3 KI as a primary standard.
If we use 50 mL burette and both solutions are 01N that means 005M solution of iodine and 01M solution of. To 500 ml of this solution add 2 g of potassium iodide and 3 ml of 2 M hydrochloric acid and titrate with the sodium thiosulphate solution using the starch solution added towards the end of the titration as an indicator until. 211 Vitex indicator laboratory grade.
Solution for standardize a sodium thiosulfate Na2S2O3 solution for a titration experiment. Standardization of 01 M Sodium thiosulfate solution Dissolve 0200 g of potassium bromate weighed accurately in sufficient water to produce 2500 ml. It is also called sodium.
Sides of the flask with 200 mL water and titrate with your thiosulfate solution until the brown iodide color becomes noticeably lighter. Mass out 6205 g of Na 2 S 2 O 3. Firstly wash out a burette twice with distilled water.
Thus iodometry is a kind of back titration.

Solved Experiment No 04 Experiment Name Standardization Of Chegg Com

0 1 N Sodium Thiosulfate Preparation And Standardization
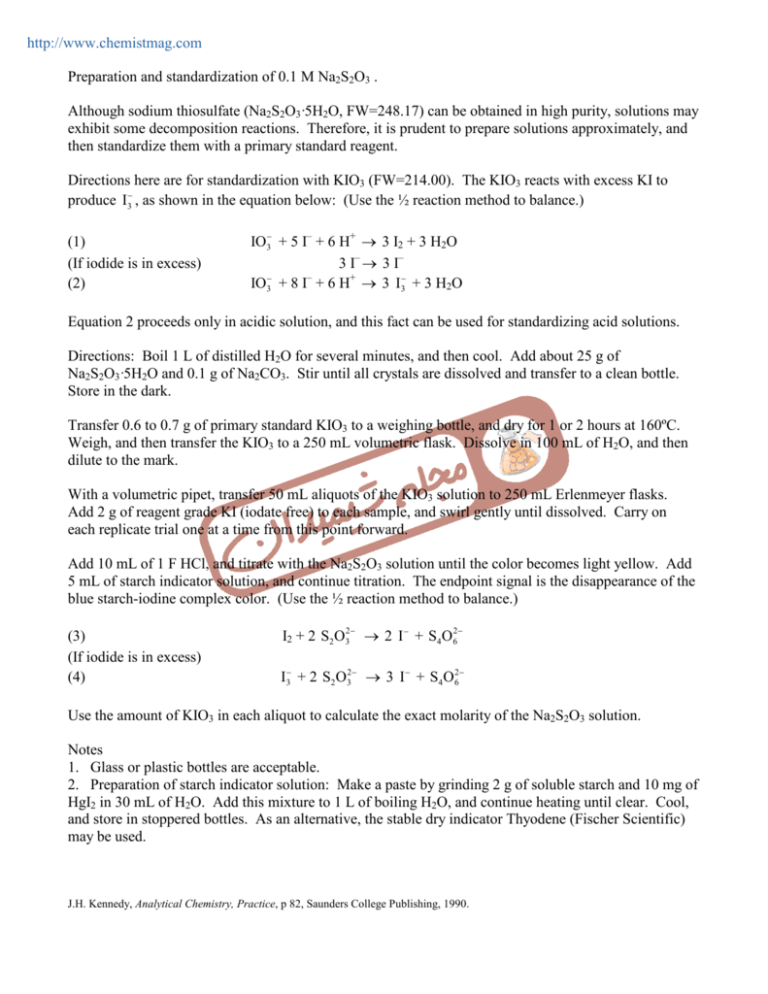
Preparation And Standardization Of 0 1 M Na2s2o3 Although

Standardization Of Sodium Thiosulphate Solution With Standard

Iodometry Titration With Sodium Thiosulfate Numerous Methods Are

Pdf E Dcapodaca Chm112l Preparation And Standardization Of A Sodium Thiosulfate Solution Ma Juryst Chelsea Armas Academia Edu
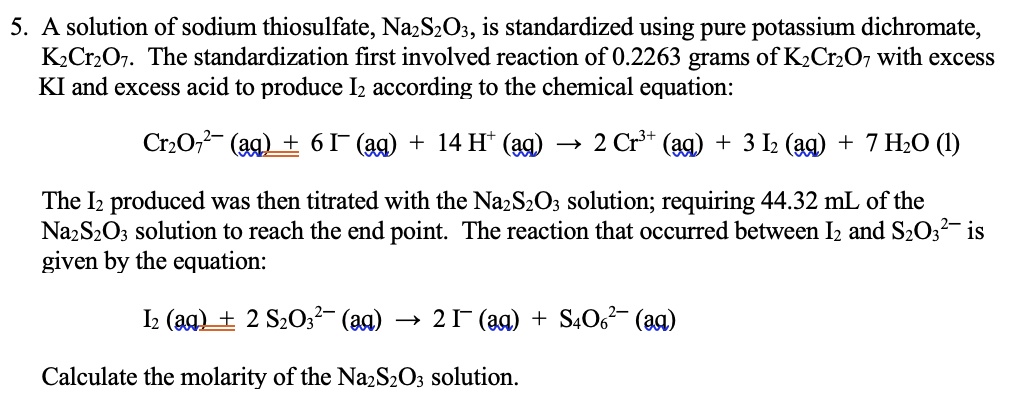
Solved 5 A Solution Of Sodium Thiosulfate Na2s2oz Is Standardized Using Pure Potassium Dichromate Kzcrzov The Standardization First Involved Reaction Of 0 2263 Grams Of Kzcrzov With Excess Ki And Excess Acid To Produce

11 Standardise A Solution Of Sodium Thiosulfate Youtube

Eureka 2017 Iodometry Redox Titration Kio3 Vs Thiosulfate Youtube
Standardize Sodium Thiosulfate According To Astm D1510 16

Preparation And Standardization Of Sodium Hydroxide Labmonk

0 1 N Sodium Thiosulfate Preparation And Standardization

Solved 18 To Standardize The Sodium Thiosulphate Solution Chegg Com

Standardization Of Sodium Thiosulfate With Potassium Dichromate Brainly In

Preparation And Standardization Of Sodium Thiosulphate 0 1n 0 1m Bpharmacynotes Pharm Analysis Youtube

Exercise 13 Preparation And Standardization Of Sodium Thiosulfate Solution Pdf Titration Chemistry
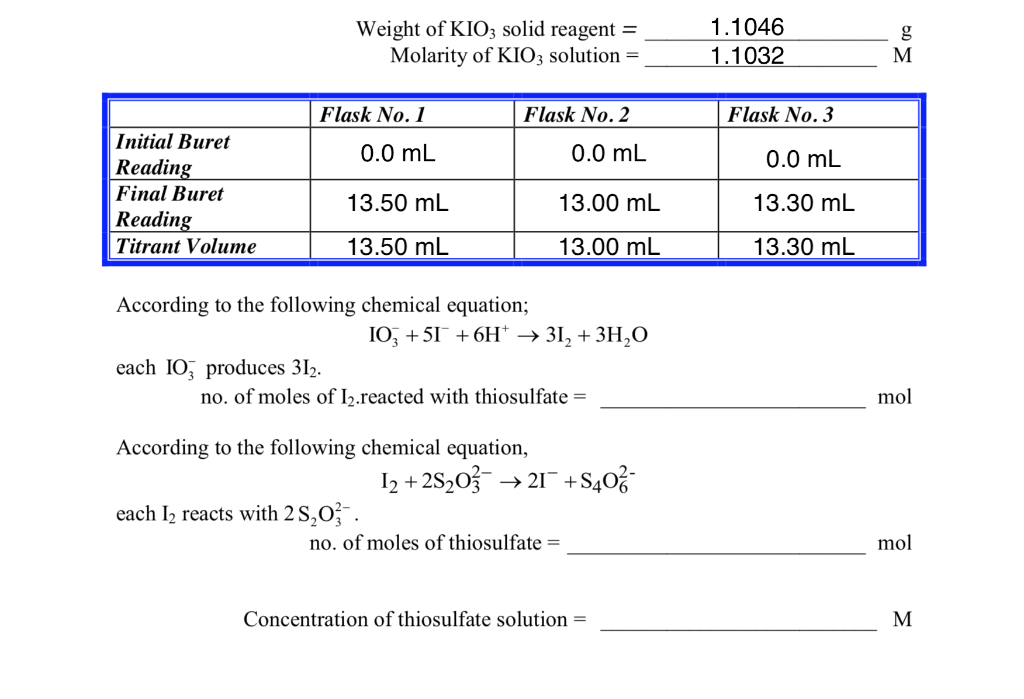
Solved Purpose Standardize A Sodium Thiosulfate Solution Chegg Com
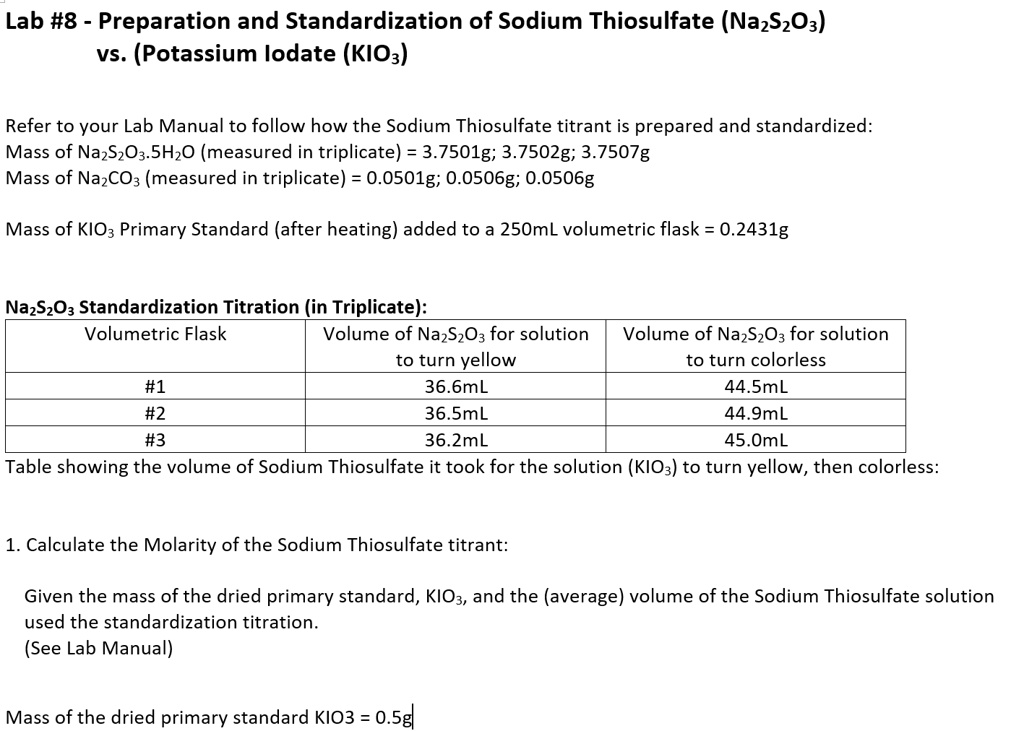
Lab 8 Preparation And Standardization Of Sodium Thios Itprospt

0 1 N Sodium Thiosulfate Preparation And Standardization
0 Response to "standardize sodium thiosulfate"
Post a Comment